This is a Phase 2/3 adaptive, double-blind, placebo-controlled study to evaluate the efficacy and safety of VX-147 for APOL1-mediated proteinuric kidney disease.
People with APOL1 genetic variants, G1 or G2, have a greater risk of developing proteinuria and chronic kidney disease, which is typically of a more aggressive course with a faster progression to end-stage kidney disease.
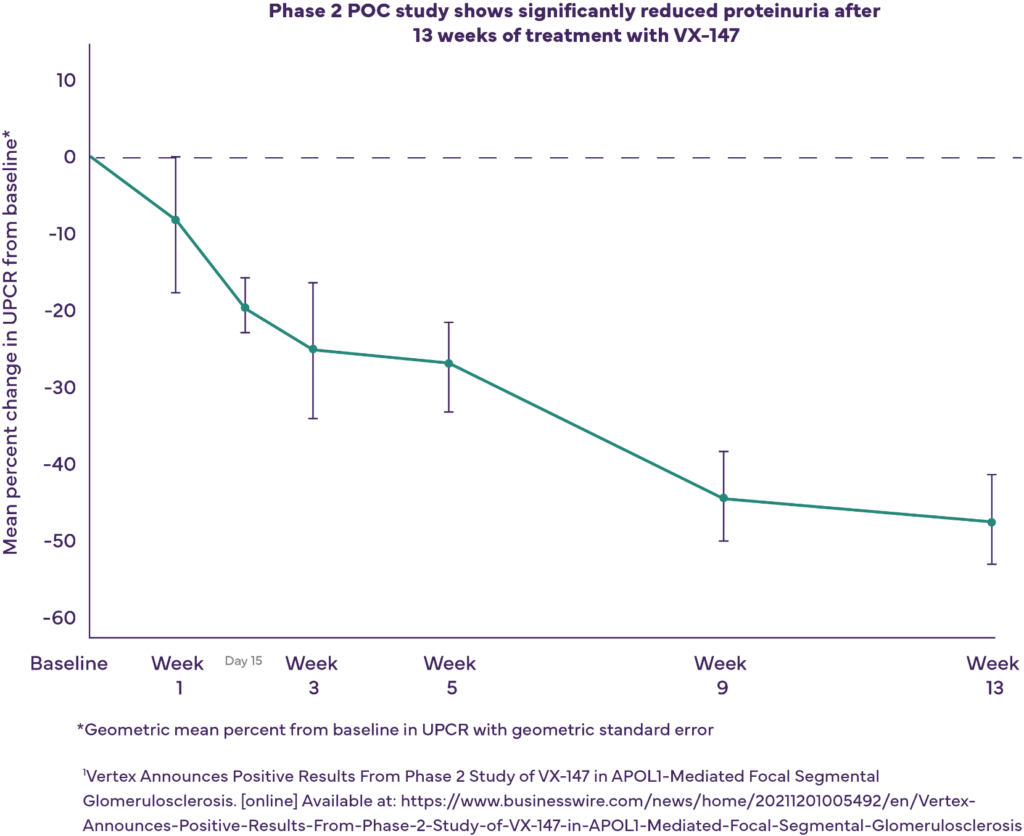
One of the main treatment goals for chronic kidney disease is to reduce or stabilize proteinuria levels. Vertex is investigating whether VX-147 will bind APOL1 and block toxic effects, leading to decreased proteinuria and improved clinical outcomes.


Many people with chronic kidney disease don’t know they have it. It’s important to know who may be at risk and raise awareness so that together, we can support them on their kidney health journey.
The APOL1 variants, G1 and G2, occur exclusively in people of African descent. Therefore, the AMPLITUDE clinical research study is recruiting people of African descent who have been diagnosed with APOL1-mediated kidney disease.
* Please note this is not a complete list of objectives and endpoints.
This allows the expansion from the Phase 2 study into a Phase 3 study, by adding additional patients. It allows for modifications of the study design for Phase 3 dependent on the outcome of Phase 2.

In a Phase 2 proof-of-concept (POC) study, Vertex found that treatment with VX-147 led to a statistically significant, substantial, and clinically meaningful mean reduction in proteinuria of 47.6% at 13 weeks compared to baseline, and was well tolerated.1